Cooperation with Swedish Innovation Authority and University to Optimize Sustainability Procedures

Sustainable and resilient business practices are important in every aspect of what we do at TSS. It is a fundamental part of our core values as well as our yearly business plans. These strategies reflect our daily work, throughout procurement, procedures, work environment, and collaboration with the community.
At TSS, we have been optimizing temperature monitoring procedures for numerous large pharmaceutical companies for over 30 years. Working closely with research communities, universities, and innovation authorities has always been a central part of our business.
Sharing best practices and lessons learned
The RESPIRE project is a collaboration between TSS AB, RISE (Research Institutes of Sweden), and KTH (Royal Institute of Technology). RESPIRE is a 3-year running project financed by Vinnova, Sweden’s innovation agency.
"We believe that collaborations like these are important to support students in their development, as well as to optimize sustainability procedures. These partnerships allow us to contribute to a more eco-conscious future. Additionally, they provide us with innovative insights that propel our product excellence forward,” said Johanna Eriksson, Chief Product Officer at TSS.
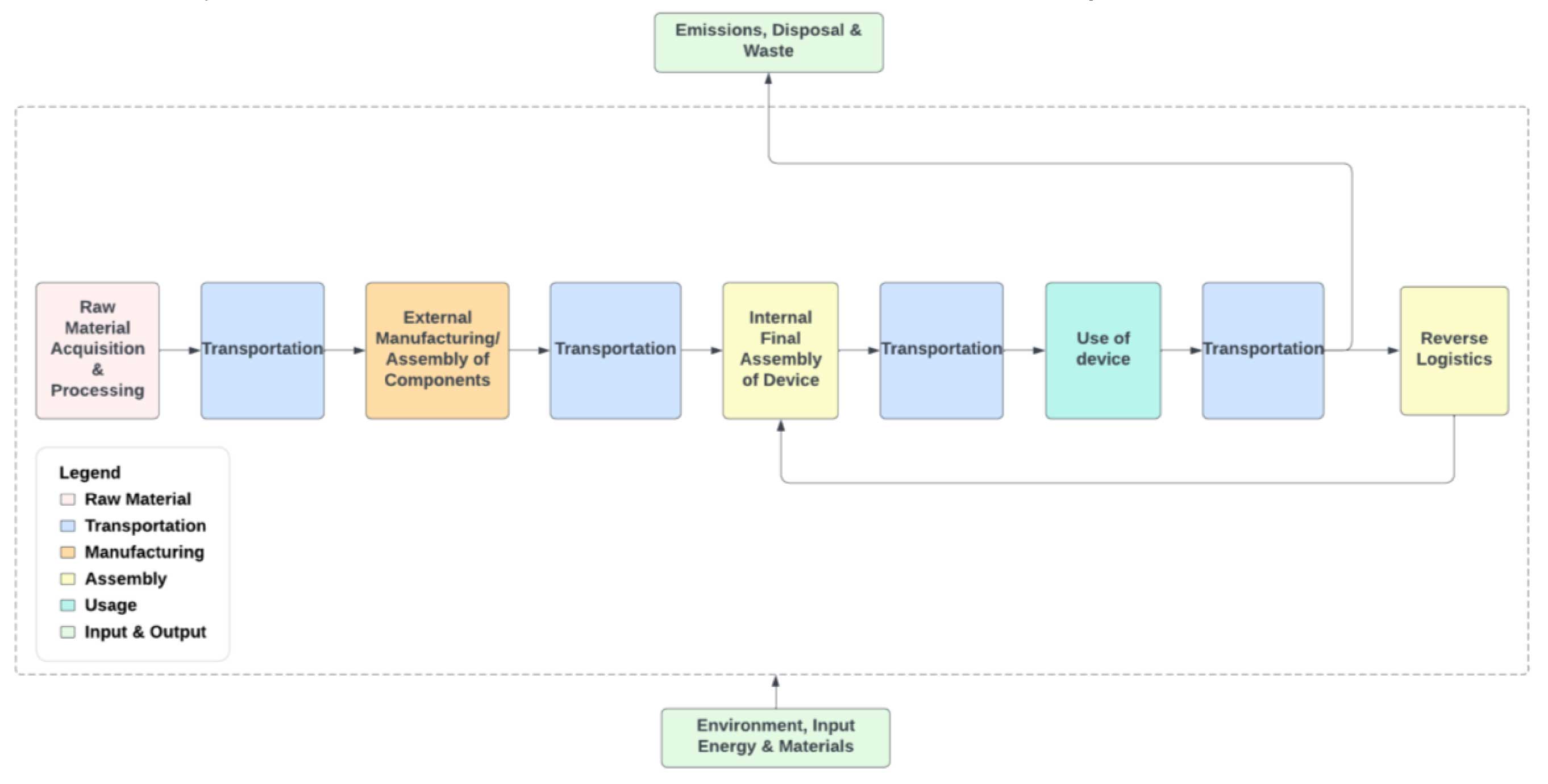
Under the RESPIRE umbrella, the master student Trupti Borade at KTH, investigated in the environmental impact of the electronic devices of TSS AB. She examined the current practices and trends in designing and producing electronic components, as well as their entire life cycle, from production to disposal. The study identified the production phase as the primary driver of the temperature loggers' environmental footprint, accounting for over 90% of the total impact.
“This significant finding underscores the need to address and optimize the environmental aspects associated with the production processes”, Trupti Borade explains. She continues: “By reviewing all materials used and considering the integration of potentially recycled content, manufacturers can minimize the impact on metal depletion, an area of paramount importance in sustainable product initiatives.”
The partnership between such industrial and research institutions can bridge the gap between theory and implementation, facilitating the transfer of innovative ideas into real-world solutions. This can lead to the development of sustainable practices, improved processes, and the creation of products and services that address critical societal and environmental challenges.
From single use to reusable devices
As the casing and PCBA (Printed Circuit Board Assembly) account for a major portion of emissions, reusing hardware through a circular business model decreases the carbon footprint significantly. Extending the lifetime of the hardware could reflect a fraction of the impact from single use devices. In a case where the units could be reused up to 16 times, the environmental impact (climate change in kg CO2-Eq) of loggers with reusable components was 90% less than the impact from single-use.
”During this study period, the most exciting part was analyzing the results obtained, and drawing conclusions based on the assessment of the temperature loggers' sustainability using the EU Taxonomy framework. It was rewarding to see how the study findings could contribute to the broader goal of promoting sustainability in the manufacturing industry and aligning with emerging regulatory requirements” said Trupti Borade.
The study highlights the significant impact of the PCBA in terms of mass and environmental footprint.
“If given the opportunity, I would like to further investigate the feasibility of using alternative materials for the PCB that are lightweight, durable, and environmentally friendly. This could involve exploring the potential of paper-based PCBs or other eco-friendly materials as alternatives to traditional PCB materials. By incorporating such materials, the environmental impact associated with resource depletion can be reduced and the overall sustainability of the temperature loggers can be improved,” Trupti Borade said.
Read the full report ≫
Learn more about our environmental strategy at TSS ≫
About Vinnova
Vinnova is Sweden's innovation agency. Our mission is to strengthen Sweden's innovative capacity and contribute to sustainable growth. We work to ensure that Sweden is an innovative force in a sustainable world.
vinnova.se/en ≫
About RISE
RISE Research Institutes of Sweden is Sweden’s research institute and innovation partner. Through international collaboration with industry, academia, and the public sector, we ensure business competitiveness and contribute to a sustainable society.
ri.se ≫
About RESPIRE
RESPIRE: Rethinking the management of unexpected events for resilient and sustainable production is a project in a collaboration between TSS AB, RISE Research Institutes of Sweden, and Kungliga Tekniska Högskolan (KTH). RESPIRE is a 3-year running project financed by Vinnova, Sweden’s innovation agency, under the strategic innovation program for Production 2030 aims for enhanced crisis management of unexpected events related to production by identifying and sharing best practices and lessons learned across sectors.
You may also be interested in

5 benefits of adopting digital temperature monitoring solutions

Join us at Temperature Control & Logistics in Düsseldorf

White paper: the future, today - the next generation of temperature monitoring
You may also be interested in

TSS Sponsors the 27th Annual Clinical Trial Supply Europe 2026 in Barcelona


