Enhancing Product Quality and Compliance at Clinical Sites

To address these challenges, pharmaceutical facilities are increasingly turning to automated temperature monitoring solutions to minimize excursions, control costs, and mitigate risks. In this article, we explore the significance of temperature monitoring in refrigerators, the impact of poor-quality fridges on product integrity, and how automated solutions offered by companies like TSS can revolutionize temperature management in pharmaceutical facilities.
The Importance of Temperature Monitoring in Pharmaceutical Refrigerators
Pharmaceutical products often require storage within specific temperature ranges to maintain their efficacy and stability. For instance, many medications must be stored between 2 to 8 °C to prevent degradation and maintain efficacy. Temperature excursions beyond these ranges can lead to irreversible damage to the products, rendering them ineffective or even harmful to patients.
Temperature monitoring in refrigerators is essential to identify and prevent excursions that could compromise product quality. Traditional manual monitoring methods are prone to human error and often lack real-time visibility, making it challenging to promptly address temperature fluctuations. Automated temperature monitoring solutions offer a comprehensive approach to tracking temperature variations, providing continuous monitoring, data analytics, and timely alerts to ensure proactive intervention when deviations occur.
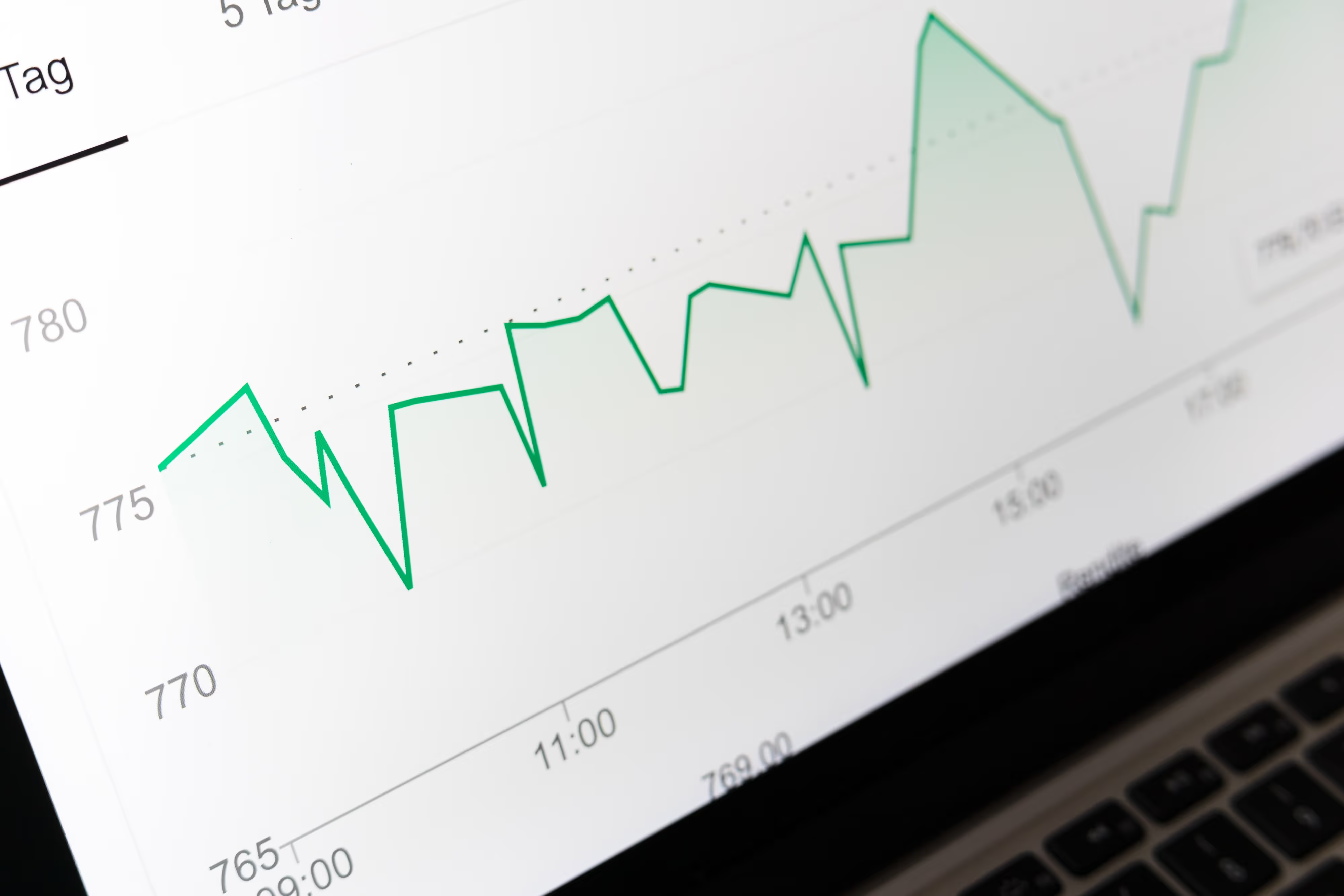
Identifying the Impact of Poor-Quality Refrigerators
The quality of refrigerators used in pharmaceutical facilities significantly impacts product integrity. A good refrigerator maintains temperature stability with minimal fluctuations, ensuring that medications remain within the specified temperature range consistently. In contrast, poor-quality fridges may exhibit wide temperature fluctuations, posing a significant risk to product stability.

A categorization system helps distinguish between good, okay, and bad refrigerators based on their temperature performance:
- Good Fridge: Maintains fluctuations within 0.5 ºC, with no peaks unless the door is opened.
- Okay Fridge: Fluctuations remain below 1 ºC, but peaks may occur during defrosting cycles.
- Bad Fridge: Exhibits fluctuations of 4.5 ºC or more, indicating significant temperature instability.
Understanding Stability Budgets in Pharmaceutical Supply Chains
The concept of stability budgets serves as a critical management tool in pharmaceutical supply chains, allocating resources to ensure product safety and quality throughout the manufacturing, packaging, storage, and distribution processes. A stability budget defines the allowable time and conditions a product can spend outside its specified storage range without compromising its integrity.
Stability budgets combine data from temperature studies and stability testing to determine the product's thermal stability and tolerance to temperature excursions. By establishing predetermined limits for temperature deviations, pharmaceutical manufacturers can prevent costly investigations, minimize product wastage, and ensure timely delivery of critical drugs to patients.
Automated Solutions for Enhanced Temperature Monitoring
At TSS, we offer automated temperature monitoring solutions designed to streamline temperature management processes and enhance product quality assurance. These solutions provide guaranteed data upload, comprehensive reports and dashboards, data analytics capabilities, and seamless monitoring through automated data feeds.
By implementing automated temperature monitoring systems, pharmaceutical facilities can achieve:
- Decreased excursions through real-time monitoring and timely alerts.
- Total cost control by identifying cost-saving opportunities and minimizing product wastage.
- Increased visibility and control over temperature conditions with comprehensive reporting and analytics.
- Simplified release processes and access to all temperature data for compliance with regulatory authorities.
- Mitigation of potential risks to the company's reputation by ensuring product integrity and compliance with quality standards.
- Periodic revision of procedures and reports to identify hidden risks or non-conformity issues.
- Automation of manual processes to reduce time spent on excursion handling and improve operational efficiency.
In the pharmaceutical industry, maintaining precise temperature control is essential for ensuring product quality, safety, and compliance with regulatory requirements. Automated temperature monitoring solutions offer pharmaceutical facilities a comprehensive approach to managing temperature variations, minimizing excursions, and enhancing product integrity.
By leveraging automated solutions, pharmaceutical facilities can achieve greater control over temperature conditions, reduce operational costs, and mitigate risks associated with temperature deviations. As the industry continues to prioritize quality assurance and regulatory compliance, investing in advanced temperature monitoring technologies becomes indispensable for safeguarding product integrity and maintaining patient safety.
The TSS Facility Application is ideal for clinical sites, facilities, and pharmacies. Automatic real-time data feed eliminates the need for manual recording of temperature, reduces the risk of data gaps and sends alerts in case of issues, thereby reducing waste-related costs and increasing patient safety.
Learn More >
You may also be interested in
.avif)
TSS Welcomes Thomas Lindén as New Chief Technology Officer

Join us at Clinical Trial Supply Europe 2022
On-demand webinar: Digitalization and its impact on the pharma supply chain
You may also be interested in

TSS Sponsors the 27th Annual Clinical Trial Supply Europe 2026 in Barcelona


