Proactive Temperature Management: The Key to a More Resilient Pharma Supply Chain

With medicine shortages affecting nearly every hospital pharmacy in Europe, the industry must rethink how it manages and prevents supply disruptions. The discussion at LogiPharma focused on how real-time temperature monitoring is transforming logistics-reducing waste, improving efficiency, and ultimately ensuring that patients receive the medications they need, when they need them.
Medicine Shortages and Supply Chain Vulnerabilities
A recent report by the European Association of Hospital Pharmacists (EAHP) revealed that 95% of hospital pharmacists across Europe have reported medicine shortages, leading to delays in care (59%), suboptimal treatment (43%), and even cancellation of care (35%).
One of the key factors contributing to these shortages is temperature excursions-when pharmaceutical products are exposed to conditions outside their required temperature range. Each year, the pharma industry loses an estimated $20-30 billion due to failures in temperature-controlled logistics.
According to the World Health Organization (WHO), up to 50% of vaccines are wasted globally, with temperature excursions playing a significant role.
The consequences go beyond financial losses-supply chain inefficiencies directly impact patient health. If a shipment is delayed or compromised, it can mean the difference between a patient receiving life-saving treatment or going without it.
From Reactive to Proactive: The Role of Real-Time Temperature Monitoring
Historically, pharma logistics relied on passive temperature monitoring-tracking excursions only after they happened. This reactive approach often led to costly investigations, reshipping of products, and increased carbon emissions from expedited transport.
Now, with real-time temperature monitoring, companies can detect and address temperature issues as they happen, preventing costly spoilage and ensuring compliance with strict pharmaceutical regulations.
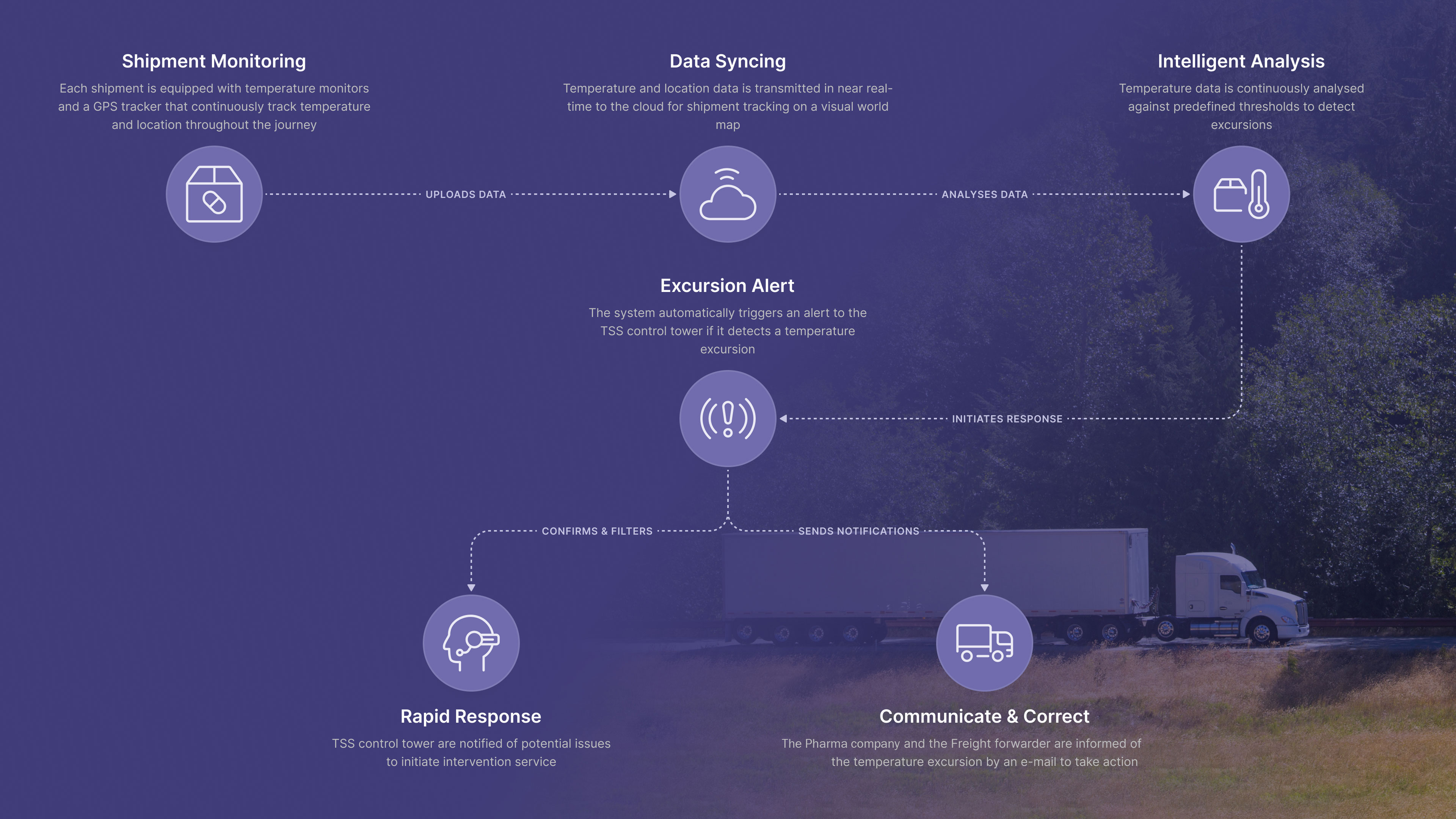
How It Works:
1. Each shipment is equipped with a temperature logger that records temperature data every 30 minutes, transmitting it in real time.
2. If a temperature deviation occurs, an alert is sent immediately to the TSS control tower.
3. To prevent false alarms, temperature changes near designated facilities are filtered out.
4. The freight partner is notified and asked to take corrective action. If no action is taken, a follow-up reminder is sent within an hour.
A Smarter, More Sustainable Future for Pharma Logistics
The shift toward real-time data and predictive analytics represents a major opportunity for the pharma industry. By embracing proactive monitoring, companies can:
- Reduce product waste
- Lower carbon emissions from emergency shipments
- Improve supply chain efficiency and resilience
- Ensure compliance with regulatory standards
Beyond financial and environmental benefits, the true impact of these advancements is on patients who rely on life-saving medicines. By optimizing cold chain logistics and preventing temperature excursions, the industry can ensure that critical treatments reach those who need them-safely and on time.
At TSS, we are committed to helping pharmaceutical companies build smarter, greener, and more resilient supply chains.
Want to learn more?Let’s continue the conversation! Contact us today to find out more about the intervention process and explore how real-time monitoring can help secure your pharmaceutical supply chain. Contact us > |
You may also be interested in

Join us at Temperature Control & Logistics in Düsseldorf

Enhancing Product Quality and Compliance at Clinical Sites
You may also be interested in

TSS Sponsors the 27th Annual Clinical Trial Supply Europe 2026 in Barcelona


