Join us at Clinical Trial Supply Europe 2024 in Barcelona

Document
Are you in pursuit of optimizing the temperature management of your clinical trials supply chain? Get inspired by experts from the field, and learn from their experiences.
Place: Barcelona, Spain | Date: 6-7 March, 2024
How to increase sponsor value
Get the opportunity to ask our customers about their experiences.
When: 4:30 PM, March 6 (day 1)
Where: Stream 2, Clinical Supply Technology and Innovation
- Boost site efficiency in temperature monitoring
- Increase patient service level
- Ensure GxP compliance
Tine Bülow Product Owner of HOT system, Clinical Supplies - Trial Product Handling, Novo Nordisk
Claes Kalderén Chief Revenue officer, TSS
We are presenting our latest innovations, together with our customers. Come join us over some temperature controlled bubbles at our booth, and discuss your challenges with our experienced temperature control team.
Booth #41
You may also be interested in

Innovation flourishes when different perspectives meet
Moving to Stockholm’s new innovation and life science cluster in Hagastaden was a conscious choice for us at TSS.
Read moreDownload Whitepaper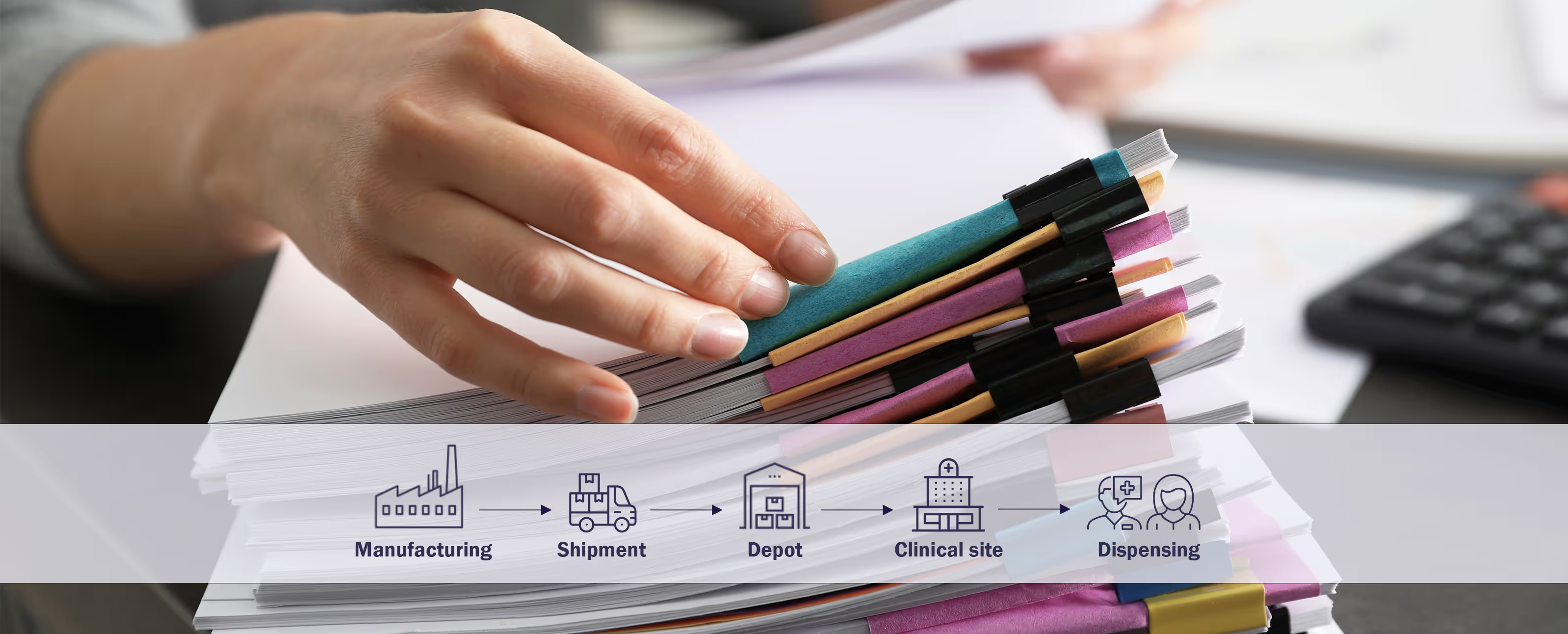
Future-Proofing Clinical Trials: How Sponsors Can Up-Scale Through Automation
At this year’s Clinical Trial Supply Europe event in Barcelona, TSS took center stage to discuss one of the most pressing challenges in clinical trial supply chains—temperature monitoring. Our Key Account Manager, Anita Leposa, highlighted how automation is revolutionizing temperature integrity management, reducing manual work, and ensuring compliance for global pharmaceutical sponsors.
Read moreDownload Whitepaper
Covid-19 situation
The effects of the Covid-19 outbreak are escalating daily and is first and foremost a human tragedy, affecting hundreds of thousands of people.
Read moreDownload WhitepaperYou may also be interested in

TSS Sponsors the 27th Annual Clinical Trial Supply Europe 2026 in Barcelona
TSS is proud to sponsor the 27th Annual Clinical Trial Supply Europe, taking place 24–25 February 2026 in Barcelona, Spain. The event brings together clinical supply professionals, sponsors, CROs, and solution providers to discuss how to strengthen resilience, quality, and patient safety across the clinical trial supply chain.
Read more
Can You Rely on the Results from Your Clinical Trials?
The success of a clinical trial depends on the reliability of its data. Many investigational drugs are sensitive to temperature, and any temperature deviation can affect their quality and, in the worst case, patient safety. Automated and integrated temperature monitoring solutions help secure data quality, support compliance, and speed up the delivery of new medicines to patients.
Read more
