How GSK Pharma in UAE optimized the supply chain through automatization

Document
Is it possible to cut costs, increase efficiency, and improve quality—all at the same time? The GSK (GlaxoSmithKline) UAE division and their main logistics company GAC (Gulf Agency Company) exceeded their expectations when they implemented temperature monitoring solutions from TSS in outbound shipments from Dubai. Additionally, they reassured the safety of what's most valuable—the patient.
The Middle Eastern region often faces significant logistics challenges in transporting temperature-sensitive products. The climatic conditions, with high temperatures and humidity levels, can quickly compromise the integrity of the products during transportation and storage, particularly pharmaceutical drugs.
These challenges require advanced temperature-controlled logistics solutions that utilize state-of-the-art temperature control and monitoring technologies to ensure the safety and efficacy of pharmaceutical products throughout their supply chain. Despite these challenges, Dubai has become an important hub for secondary distribution of pharmaceuticals to the Middle Eastern, as well as the Asian, regions.
Automating manual processes and digitizing documentation
Read the Case Study to learn more about the successful temperature monitoring process in Dubai ≫
You may also be interested in

Visibility and automation: reducing disruption due to temperature excursions
The Covid-19 pandemic has placed a global spotlight on pharmaceuticals and the importance of temperature control. The large-scale vaccination programs being rolled out across the world depend on operators and distributors being able to monitor and manage temperatures effectively. It has also shown just how challenging this can be.
Read moreDownload Whitepaper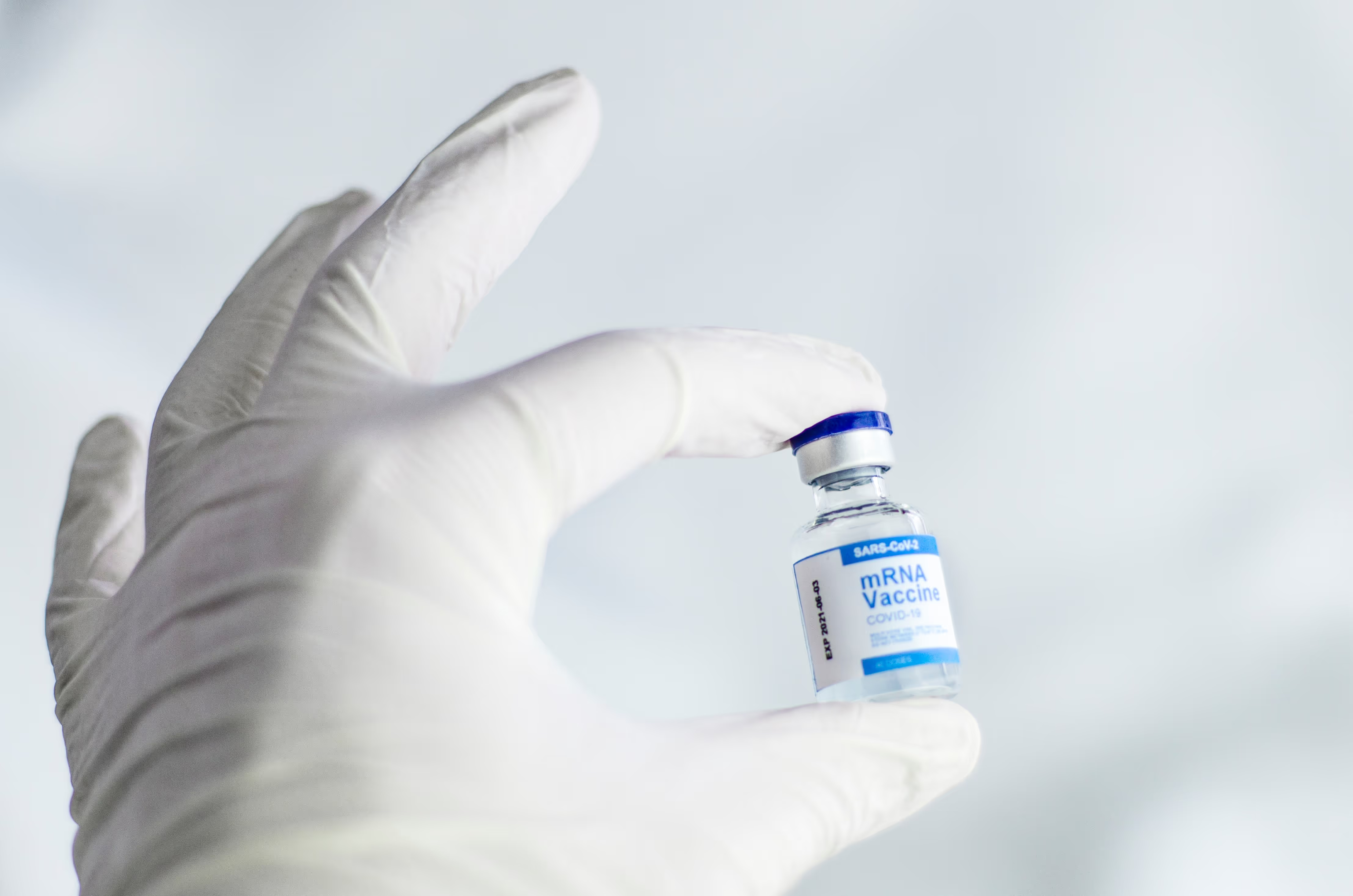
Three approaches for automating clinical supply chains
Our North America VP Evan Hahn, recently presented a webinar where he outlined three different approaches for automating clinical supply chains.
Read moreDownload Whitepaper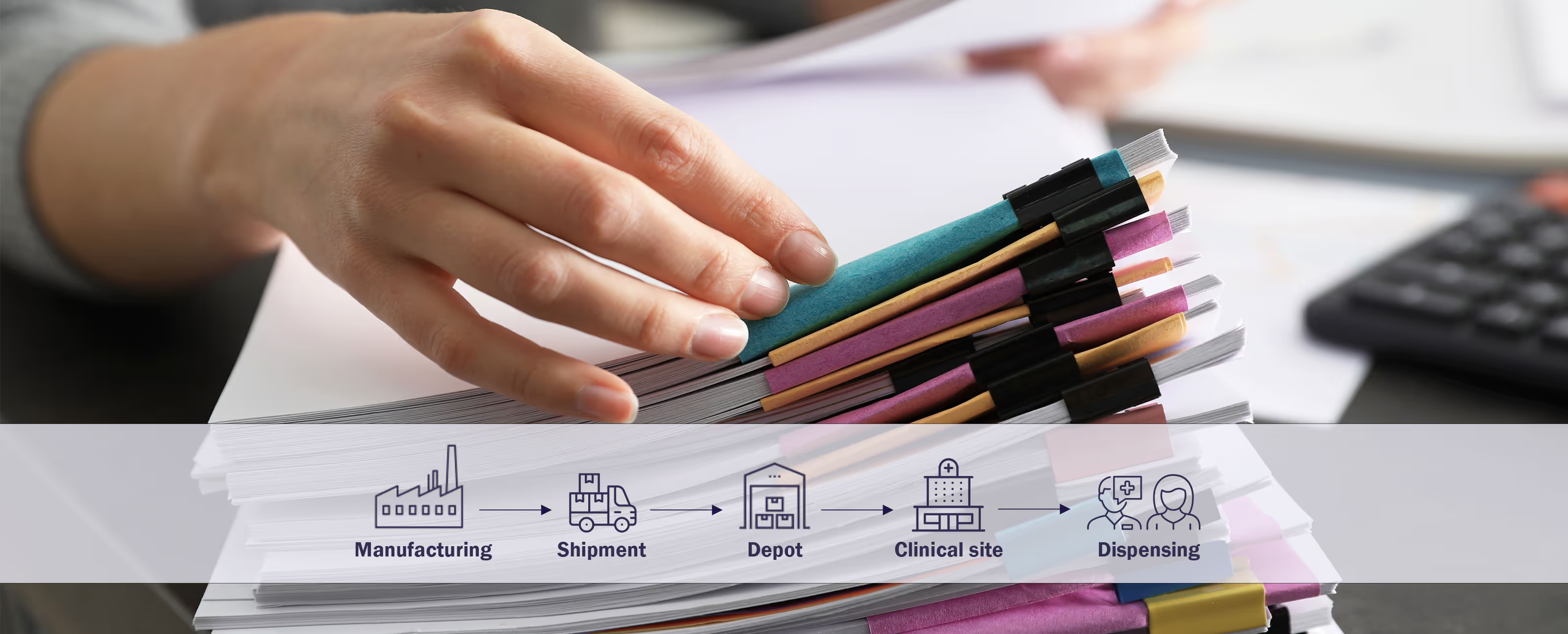
Future-Proofing Clinical Trials: How Sponsors Can Up-Scale Through Automation
At this year’s Clinical Trial Supply Europe event in Barcelona, TSS took center stage to discuss one of the most pressing challenges in clinical trial supply chains—temperature monitoring. Our Key Account Manager, Anita Leposa, highlighted how automation is revolutionizing temperature integrity management, reducing manual work, and ensuring compliance for global pharmaceutical sponsors.
Read moreDownload WhitepaperYou may also be interested in

TSS Sponsors the 27th Annual Clinical Trial Supply Europe 2026 in Barcelona
TSS is proud to sponsor the 27th Annual Clinical Trial Supply Europe, taking place 24–25 February 2026 in Barcelona, Spain. The event brings together clinical supply professionals, sponsors, CROs, and solution providers to discuss how to strengthen resilience, quality, and patient safety across the clinical trial supply chain.
Read more
Can You Rely on the Results from Your Clinical Trials?
The success of a clinical trial depends on the reliability of its data. Many investigational drugs are sensitive to temperature, and any temperature deviation can affect their quality and, in the worst case, patient safety. Automated and integrated temperature monitoring solutions help secure data quality, support compliance, and speed up the delivery of new medicines to patients.
Read more
